- Client
Health Service Executive (HSE) - Value
€40 million
GemElliott Construction Ltd were appointed as the main contractor to successfully deliver Phase 2 of Our Lady’s of Lourdes Drogheda (OLOL2).
The existing hospital is a live acute hospital in the North East with an emphasis on Orthopaedics. The old part of the hospital was constructed in 1957 and Phase 1 of the reconstruction was completed in 2006.
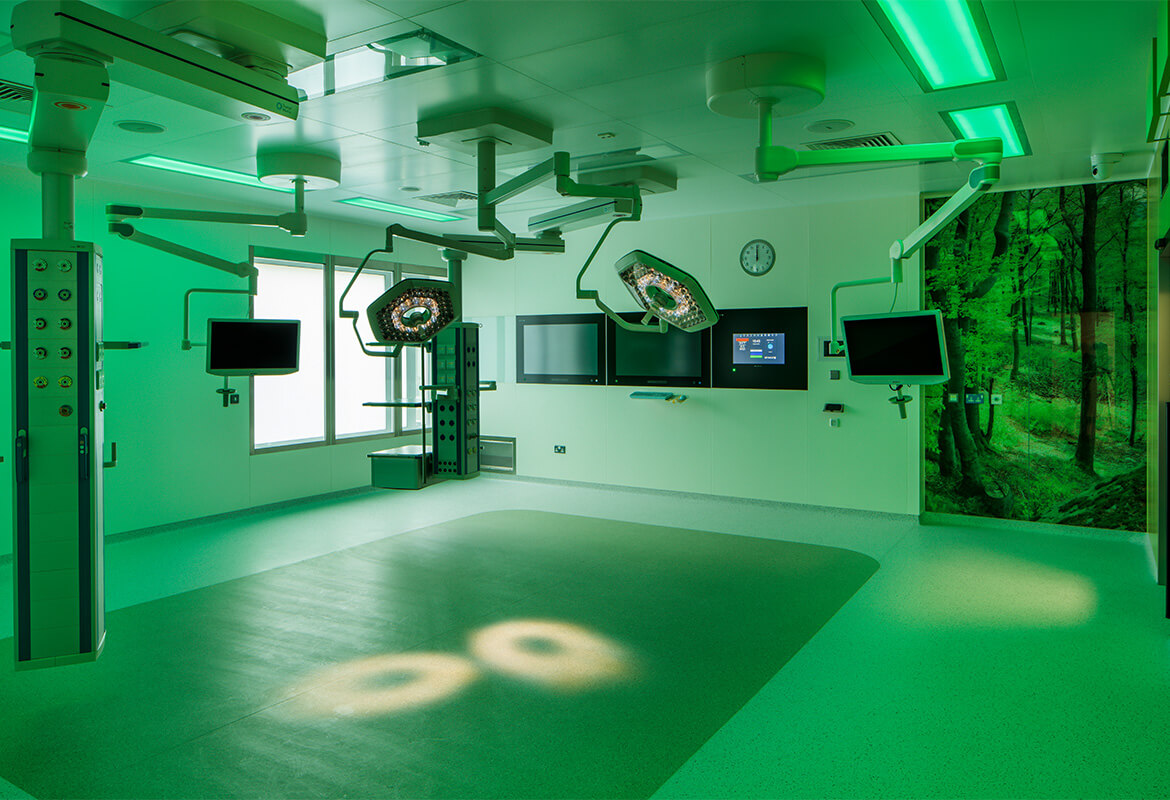
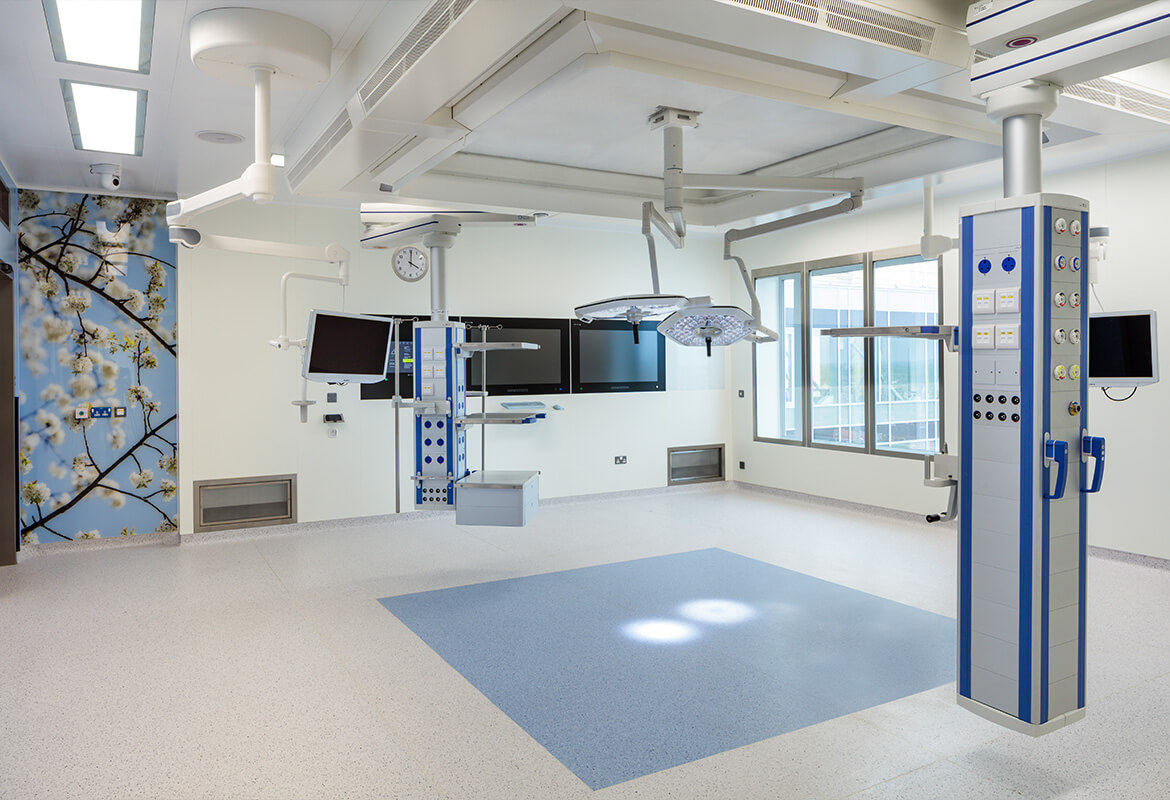
Phase 2 will consist of A&E at the ground floor level, and Ward beds at levels 2,3 &4. Level 5 will be operating theatres. All plant is at roof level both indoors and outdoor.
The project was awarded in February 2016 after a restricted public procurement process. Prior to commencement, we agreed on a schedule of interface works with the Hospital and these works did not start until each method statement went through a strict approval process. Extensive enabling works had to take place prior to any construction works. This included installing a 6 storey dust screen in front of the hospital as part of the aspergillus prevention measures. Temporary retaining works were carried out to existing services and a di-version of Foul, ESB, medical gases and coms had to be undertaken before any foundations started. Toilets on the ground floor of the existing A&E department had to be relocated as part of the enabling works. We also installed 1 hour fire rated partition at each level to protect the existing hospital from dust and fire at each tie location.
Once the RC frame was completed we commenced with the blockwork infill external wall panels. The entire facade of the building was made up of Argeton Terracotta rain screen and Schucco curtain walling. Once the blockwork was completed we commenced with the setting out of the brackets for the rain screen and the installation of the vapour membrane and insulation. The panel support rails were fitted in conjunction with the windows being installed. Once the windows were installed the Terracotta panels and flashing were fitted. Prior to the Terracotta panel being fitted a void closure process was undertaken to prove compliance. A structural steel bridge link was also constructed over the existing hospital and linked Phase two with Levels 6, 7 & 8 within the existing hospital. The link was then cladded in Structural Curtain walling.
These works were very difficult from a health and safety point of view due to the height and the restricted location of the link. Extensive temporary works took place prior to the installation of this link. Fitted furniture and flooring details were all workshopped with the hospital’s infection control team. Once areas become completed we carried out snagging of same using Site works. These are tracked and areas are locked off.
A commissioning programme is developed will all stakeholders 3 months from completion. This is tracked on a weekly basis. Once all testing and commissioning is completed the Design Team in conjunction with the Estate team are asked to attend a verification of com-missioning review. Once this is completed, the end user will be invited to demonstrations and all of these will be documented.
The O&M manual is worked on months from completion and all of the technical submissions information is stored for use in the O&M. The format is agreed with the Design Team and our full time on site QHSE officer keeps the O&M up to date on a constant basis. BCAR certs are also requested from the Supply chain early in the process to ensure an early BCAR compliance. Where possible we issue an early 21 validation notification to enable the end user to use the building as quickly as possible.
This project is a fine example of our ability and expertise in executing an Acute Healthcare project in a live Acute Hospital Campus.
-
Building Type
Acute Hospital Campus -
Scale
9000m2 -
Duration
24 months -
Architect
Wejchert Architects -
Services Engineer
Varmings Consulting Engineers
Project Gallery
Get in touch
At Elliott Group, we are proud of our exceptional team of professionals who are available to talk to you in person or who can answer any question you might have.